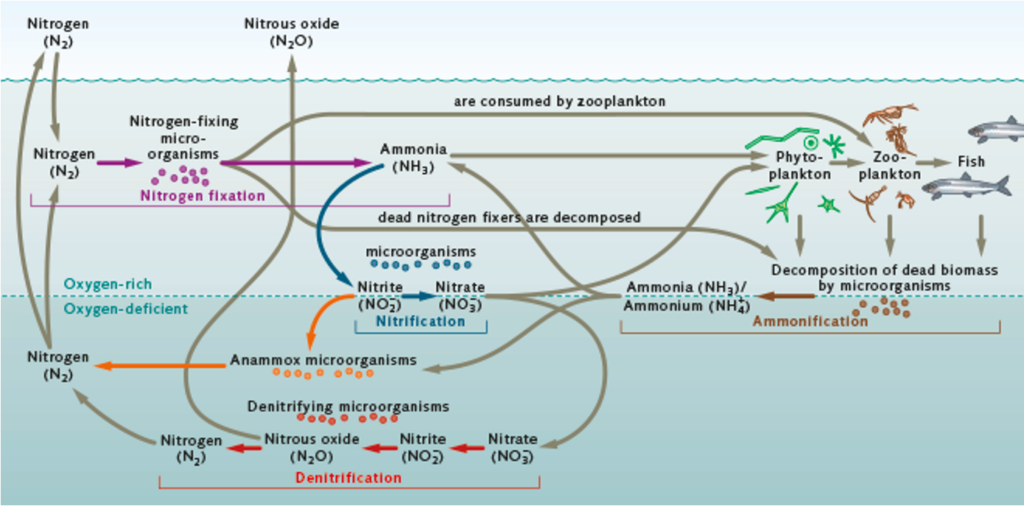
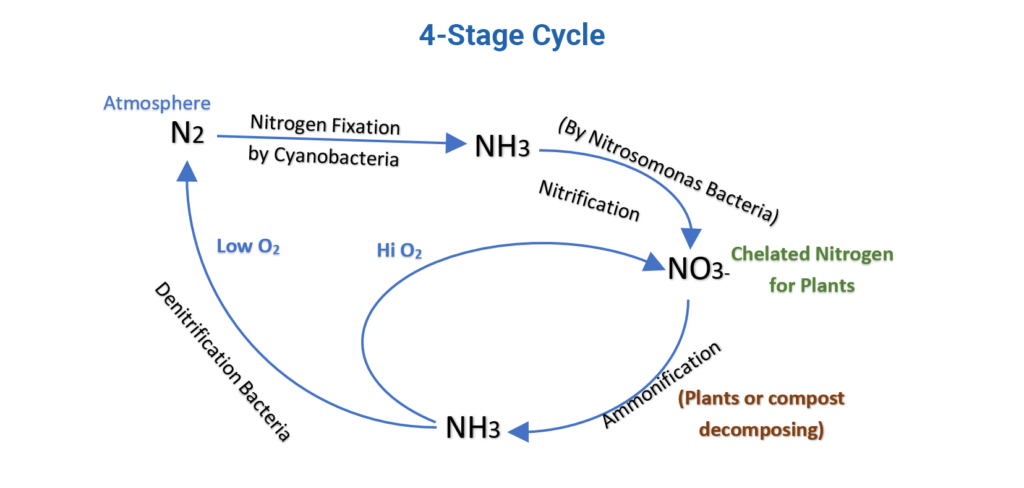
Nitrogen: why is 78% of our atmosphere made up of the stuff, yet it is expensive and problematic to get it into our fields! Click this World Ocean Review article to read more about how the ocean transforms Nitrogen from an inert, unusable gas (N2), to a chelated form for plant uptake (NH3, NH4, NO2– and NO3–), a process called Nitrification performed by Cyanobacteria and Nitrosomonas bacteria. One observation of the cycle is that Nitrogen conversion will cycle between forms of NH3 and NO3– (good for plants) in oxygen-rich environments, and Nitrogen will undergo denitrification back to N2 in oxygen-deficient environments. That’s why aerators are so important for compost teas, and compost piles should be turned.
Here’s a very simple 4-stage Cycle:
Research from World Ocean Review
Research taken from: https://worldoceanreview.com/en/wor-5/living-with-the-coasts/coastal-functions/transformations-of-a-key-nutrient-the-nitrogen-cycle/
“Nitrogen is a nutrient vital to plant growth. In the environment, nitrogen undergoes several conversions in a natural cycle through biological processes along various pathways. Gaseous atmospheric nitrogen is largely converted into nitrogen compounds by microorganisms; this makes these compounds available to macro-algae, phytoplankton and terrestrial plants. When the biogenic nitrogen compounds are converted back into gaseous nitrogen the cycle is completed.
Elementary (pure) nitrogen (N2) constitutes 78 percent of the Earth’s atmosphere, but this element cannot be used by plants directly. However, a number of microorganisms are capable of using gaseous nitrogen. These organisms include the marine cyanobacteria (formerly termed blue algae) as part of the phytoplankton and the terrestrial rhizobia associated with legumes such as beans. They can take up pure nitrogen and convert it into ammonia (NH3) or ammonium ions (NH4+). This process is called nitrogen fixation. In the further course of the cycle, the ammonia or ammonium is taken up by other groups of microorganisms, and especially by Nitrosomonas bacteria, which convert it into nitrite ions (NO2–) and ultimately into nitrate ions (NO3–). This conversion process is called nitrification. These ions can be taken up directly by macro-algae, phytoplankton and terrestrial plants which use it to assemble amino acids among other organic compounds. When they die, their dead biomass is decomposed by a multitude of other microorganisms.
Along with the rest of the biomass, the nitrogen compounds contained in the macro-algae, phytoplankton and terrestrial plants are also decomposed, primarily through a process called ammonification. As part of this process, the nitrogen compounds contained in dead biomass, such as the amino acids, are converted back into ammonia and ammonium. The ammonia and ammonium are then once again available for nitrification by microorganisms, and by Nitrosomonas in particular. The nitrogen cycle is completed by a process of decomposition called denitrification, which ultimately is also of particular importance to keeping waters clean. Nitrate ions (NO3–) contained in water are converted directly into elementary nitrogen (N2) as well as nitrous oxides (NO and N2O) by microorganisms (denitrifying bacteria). As a result, terrestrial plants and the majority of phytoplankton can no longer use these substances as nutrients.”